What Is Addition Elimination Reaction With Example
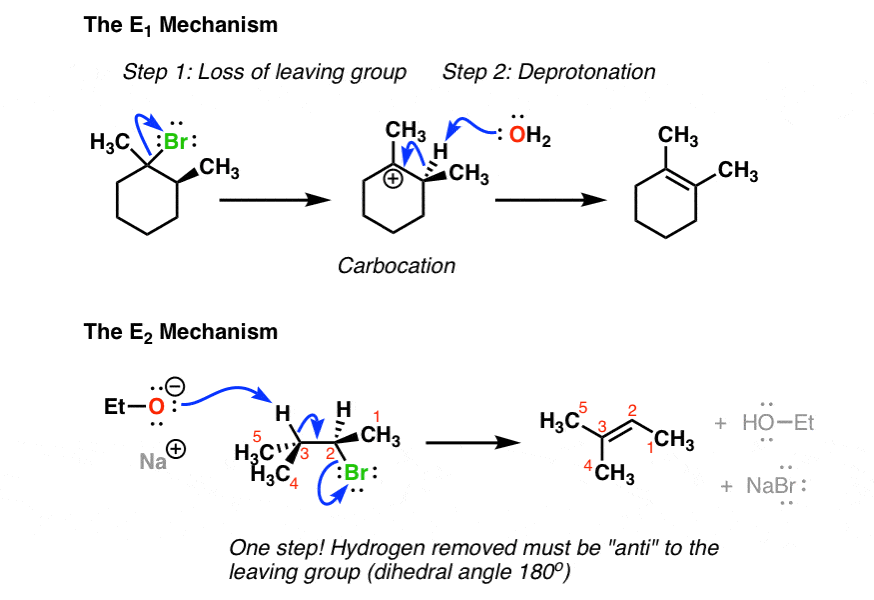
In E2 elimination shows a second order rate law and occurs in a single concerted step proton abstraction at C α occurring at the same time as Cβ-X bond cleavage. The overall mechanism of an addition-elimination reaction is known as an addition-elimination mechanism.
The E1 Reaction And Its Mechanism Master Organic Chemistry
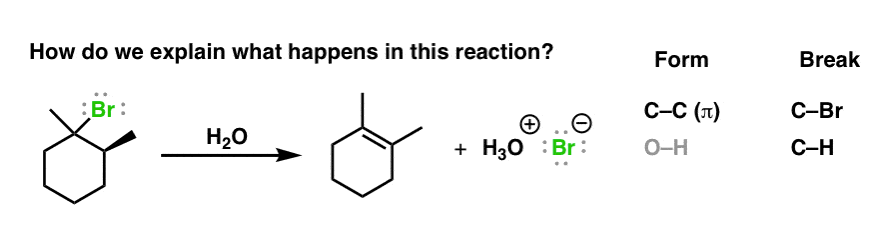
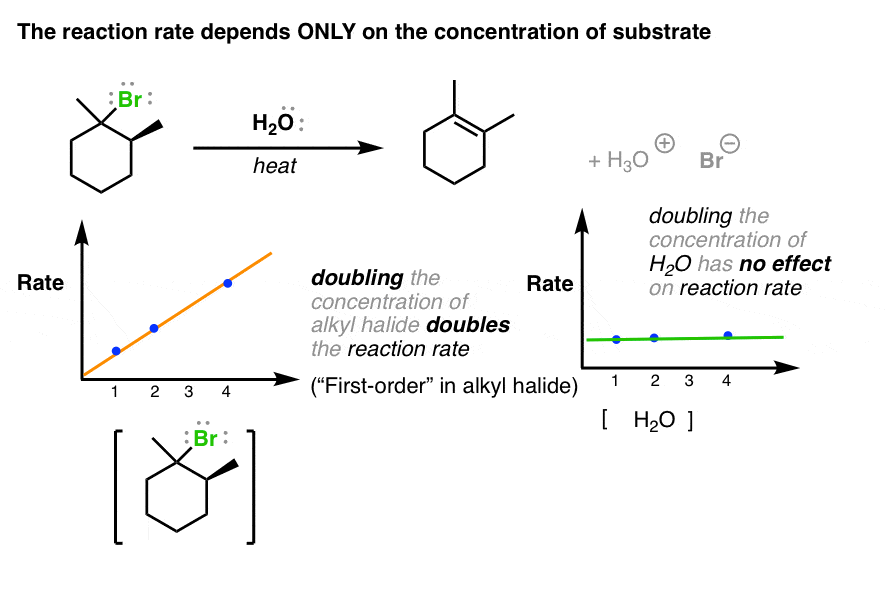
Unimolecular elimination reactions also called E1 use a different mechanism that involves a carbocation.

What is addition elimination reaction with example. Propene is not the only product of this reaction however the ethoxide will also to some. Addition reactions occur with unsaturated compounds. The general equation for an elimination reaction.
A reaction where the reactant is broken down into one or more product is called an elimination reaction. TextAtextB to textC Notice that C is the final product with no A or B remaining as a residue. The key difference between oxidative addition and reductive elimination is that oxidative addition refers to the addition of two anionic ligands to a metal complex whereas reductive elimination refers to the removal of two anionic ligands from a metal complex.
The SNAr addition-elimination mechanism. When both leaving atoms are halogens the reaction is known as dehalogenation. ADDITION-ELIMINATION REACTIONS OF ALDEHYDES AND KETONES This page looks at the reaction of aldehydes and ketones with 24-dinitrophenylhydrazine Bradys reagent as a test for the carbon-oxygen double bond.
It tends to be paired with reductive elimination the reverse reaction if and only if the added ligands were cis otherwise it is a separate reaction that. It also looks briefly at some other similar reactions which are all known as addition-elimination or condensation reactions. Since primary carbocations rarely form elimination.
Oxidative addition is typically an organometallic chemistry reaction wherein a molecule adds across a square-planar transition metal complex to form an octahedral complex. Examples of addition reactions include the reaction between ethene and bromine polymerisation reactions and hydrogenation reactions. E1 and E2 reactions in the laboratory.
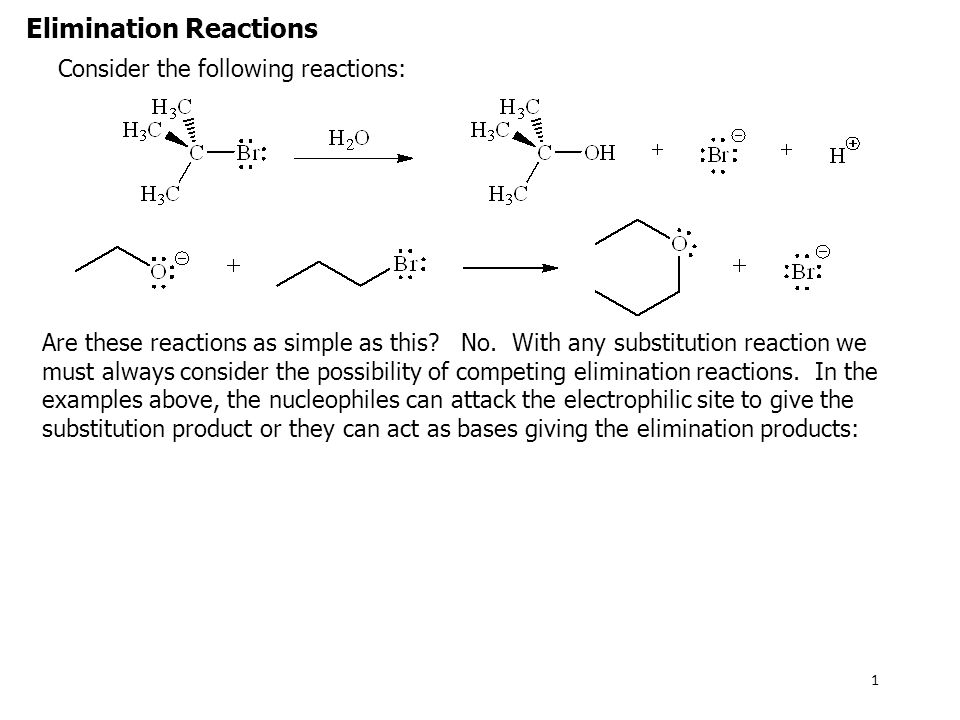
There are 6 nucleophilic substitution mechanisms encountered with aromatic systems. The general equation for an addition reaction. E2 elimination reactions in the laboratory are carried out with relatively strong bases such as alkoxides deprotonated alcohols OR.
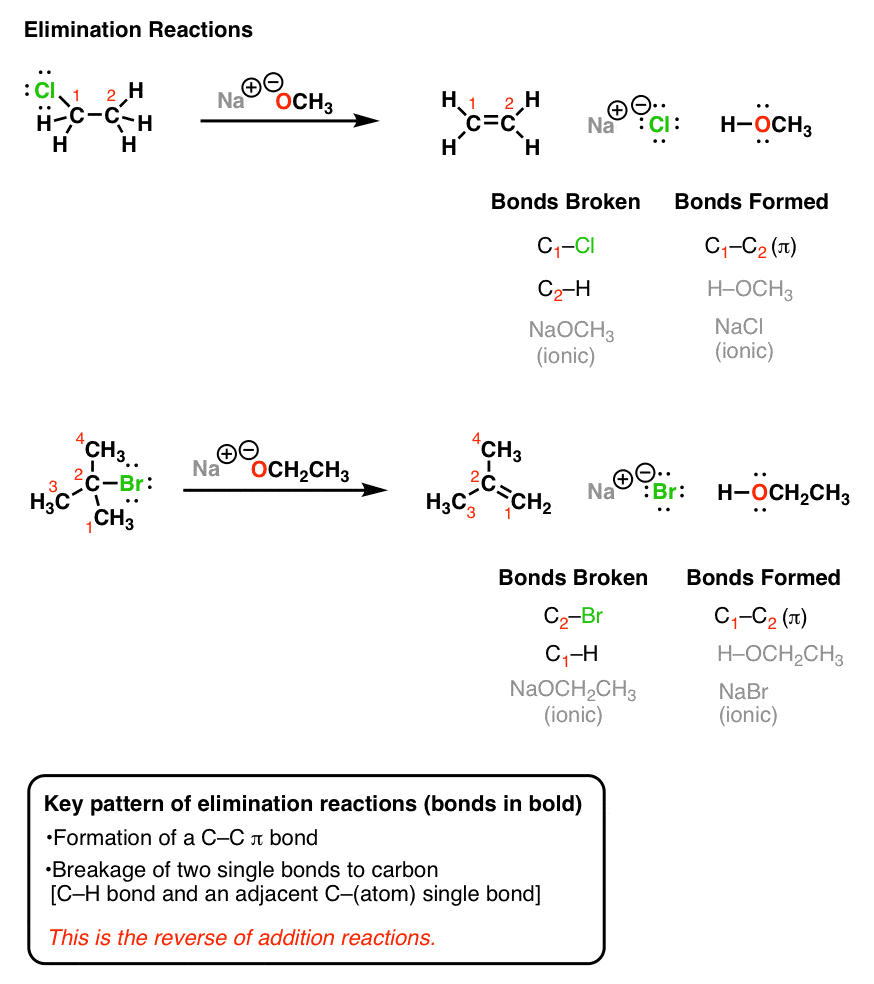
Imagine that you are given two sets of wooden planks and asked to make something out of them - anything you want. Oxidative addition and reductive elimination are chemical reactions that are opposite to each other. Base removes a proton from the β-carbon atom while the halogen atom leaves from the α-carbon resulting in the formation of a π-bond.
2 reactions have some features in common as do E1 and S N 1 reactions. Such eliminationseliminations areare alsoalso calledcalled β-elimination reactions. The removal of a hydrogen atom and a halogen atom for example is known as dehydrohalogenation.
Elimination reactions are commonly known by the kind of atoms or groups of atoms leaving the molecule. 2-Bromopropane will react with ethoxide for example to give propene. Alkyl halides undergo elimination via two common mechanisms known as E2 and E1 which show some similarities to S N 2 and S N 1 respectively.
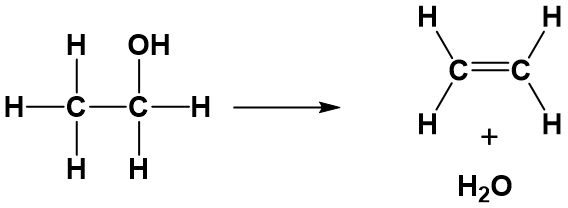
Elimination reactions occur with saturated compounds. When both leaving atoms are halogens the reaction is known as dehalogenation. The simplest examples of this class of reactions.
The removal of a hydrogen atom and a halogen atom for example is known as dehydrohalogenation. In general most of the elimination reactions involve the loss of at least one hydrogen atom to form the double bond. Some nucleophilic aromatic substitution reactions occur via a two-step mechanism in which the first step by definition is an addition and the second step an elimination.
Elimination reactions are commonly known by the kind of atoms or groups of atoms leaving the molecule. Elimination reaction s can be treated formally as the reverse of additions. A nucleophilic aromatic substitution is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group such as a halide on an aromatic ring.
You should see this in an advanced inorganic chemistry class. Alcohol dehydration and ethane cracking are examples of elimination reactions. When the reaction occurs in a single step mechanism it is known as E2 bi-molecular reaction reaction and when it has a two-step mechanism it is known as E1 unimolecular reaction reaction.
An elimination reaction occurs when a reactant is broken up into two products.
Ch105 Chapter 8 Alkenes Alkynes And Aromatic Compounds Chemistry
Elimination Reactions Ppt Download

Introduction To Elimination Reactions Youtube
Difference Between Addition And Substitution Reactions Definition Types Characteristics Examples Comparison
The E1 Reaction And Its Mechanism Master Organic Chemistry

Elimination Reaction Definition Examples Mechanism And Applications

Addition Elimination And Substitution Reactions Siyavula Textbooks Grade 11 Physical Science Openstax Cnx
E1 Vs E2 Comparing The E1 And E2 Reactions Master Organic Chemistry
Introduction To Elimination Reactions Master Organic Chemistry
Illustrated Glossary Of Organic Chemistry Beta Elimination

Addition Reaction Definition Examples And Mechanism

8 5 Elimination Reactions Organic Chemistry 1 An Open Textbook

Elimination Reaction Definition Examples Mechanism And Applications

8 5 Elimination Reactions Organic Chemistry 1 An Open Textbook

Addition Reaction Definition Examples And Mechanism

Elimination Reaction Definition Examples Mechanism And Applications